What is Assisted Reproductive Technology (ART)?
Assisted Reproductive Technology (ART) refers to medical techniques used to help individuals or couples conceive a child by manipulating eggs, sperm, or embryos outside the body.
Which are the possible ART options?
Common ART options include In Vitro Fertilization (IVF), Intracytoplasmic Sperm Injection (ICSI), Intrauterine Insemination (IUI), use of donor eggs or sperm, and gestational surrogacy.
Eggs are fertilized with sperm in a lab, and resulting embryos are transferred to the uterus. Best for tubal factor infertility, endometriosis, poor ovarian reserve, or unexplained infertility.
b) Intracytoplasmic Sperm Injection (ICSI):
A single sperm is directly injected into an egg to assist fertilization, often used in severe male infertility.
c) Intrauterine Insemination (IUI):
Washed and concentrated sperm are placed directly into the uterus around the time of ovulation. Often used for mild male infertility or ovulation issues.
d) Donor Eggs or Sperm:
Eggs or sperm from a donor are used when one partner’s gametes are unavailable or unsuitable. Recommended when one partner lacks viable gametes or carries a genetic condition. Ensures a healthy embryo and successful conception.
e) Gestational Surrogacy:
An embryo created via IVF is implanted in a healthy surrogate who carries the pregnancy on behalf of the intended parents. Needed when the intended mother cannot carry a pregnancy due to uterine issues, medical risk, or absence of uterus.
Infertility conditions where ART is often the only viable solution
1. Severe Male Factor Infertility
- Disorders: Azoospermia (non-obstructive or obstructive), severe oligospermia, genetic disorders causing sperm defects, ejaculatory dysfunction.
- ART Method: Intracytoplasmic Sperm Injection (ICSI) combined with In Vitro Fertilization (IVF).
- In cases of azoospermia, surgical sperm retrieval techniques like TESE (Testicular Sperm Extraction) or PESA (Percutaneous Epididymal Sperm Aspiration) may be necessary.
General ART Process - Treatment Pathways & Decision Points
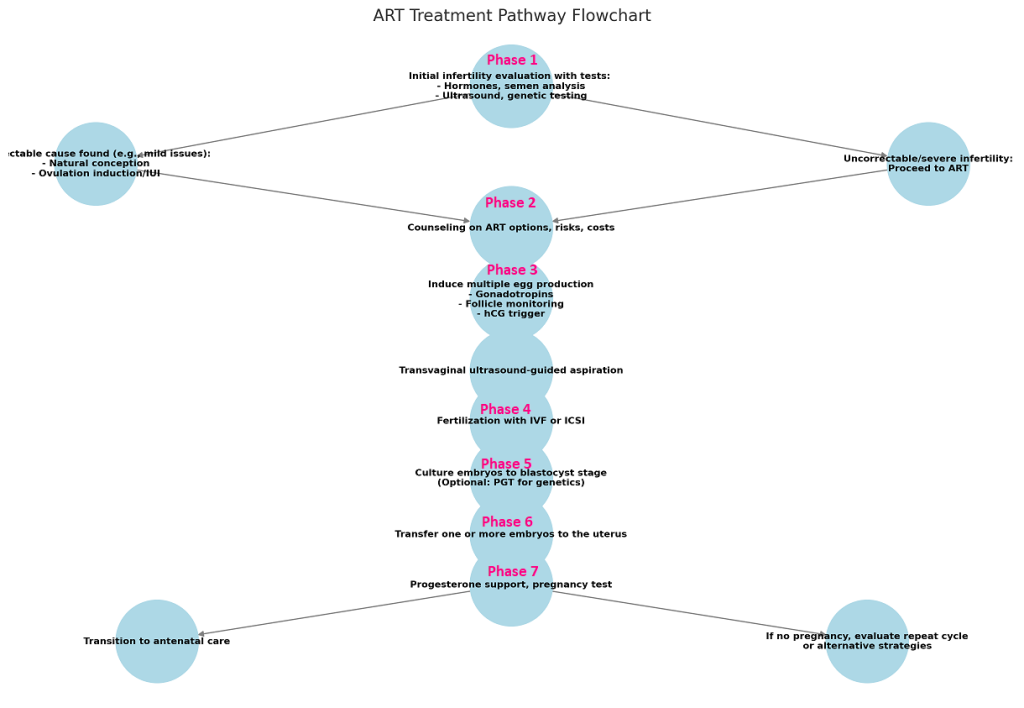

Initial Assessment
- If correctable cause found (e.g., mild endometriosis, mild male factor), attempt natural conception or simpler interventions like ovulation induction/IUI.
- If uncorrectable or severe factor infertility, proceed to ART.
For the Female Partner:
- Menstrual history: Cycle regularity, duration, and flow.
- Obstetric history: Previous pregnancies, miscarriages, or complications.
- Gynecological history: Diagnosed conditions like PCOS, endometriosis, fibroids, or pelvic inflammatory disease.
- Past surgeries: E.g., tubal surgeries, ovarian cystectomy, or uterine procedures.
- Lifestyle factors: Smoking, alcohol consumption, stress levels, and exercise routines.
- Age: Particularly important as age significantly impacts ovarian reserve and egg quality.
For the Male Partner:
- Reproductive history: Any known issues like azoospermia, erectile dysfunction, or varicocele.
- Previous surgeries: Testicular surgeries, hernia repair, or vasectomy.
- Lifestyle factors: Smoking, alcohol, drug use, or exposure to environmental toxins.
- Occupation: Heat exposure or contact with chemicals (e.g., in certain professions) affecting sperm quality.
For the Female Partner:
- Assess overall health: BMI, signs of hormonal imbalances (e.g., hirsutism, acne, or obesity in PCOS).
- Pelvic examination: Evaluate for signs of infection, masses, or tenderness indicating underlying conditions.
For the Male Partner:
- Examine testicular size, consistency, and location.
- Look for signs of varicocele, genital infections, or physical abnormalities.
For the Female Partner:
- Hormonal Tests:
- Follicle-stimulating hormone (FSH): To assess ovarian reserve.
- Anti-Müllerian Hormone (AMH): A more accurate indicator of ovarian reserve.
- Luteinizing hormone (LH): Evaluates ovulation.
- Thyroid function tests (TSH, free T4): Thyroid dysfunction can affect fertility.
- Prolactin: Elevated levels may interfere with ovulation.
- Imaging:
- Ultrasound (USG):
- Transvaginal ultrasound to evaluate uterine structure, endometrial thickness, and antral follicle count (AFC).
- Hysterosalpingography (HSG):
- Assess tubal patency and uterine cavity shape.
- Sonohysterography (if HSG is inconclusive): For detailed uterine cavity evaluation.
- Ultrasound (USG):
- Other Tests:
- Ovarian function tests: E.g., estradiol levels during the follicular phase.
- Endometrial biopsy (if indicated): To evaluate endometrial receptivity.
For the Male Partner:
- Semen Analysis:
- Evaluate sperm count, motility, morphology, and volume.
- Identifies severe male factor infertility (e.g., oligospermia, azoospermia).
- Hormonal Tests:
- FSH, LH, testosterone: To identify testicular dysfunction or hormonal imbalances.
- Genetic Testing (if indicated):
- Karyotyping, Y chromosome microdeletions, or CFTR gene mutations (for azoospermia).
- Scrotal Ultrasound (if indicated):
- To identify varicocele, testicular tumors, or other abnormalities.
Emotional Health:
- Assess stress, anxiety, or depression levels, as infertility can take a psychological toll.
- Provide referrals for counseling or support groups if needed.
Relationship Dynamics:
- Assess how infertility has affected the couple's relationship.
- Encourage open communication and shared decision-making.
Diagnosis:
- Categorize infertility as male factor, female factor, combined, or unexplained.
- Identify underlying causes (e.g., tubal blockage, low ovarian reserve, or poor sperm quality).
Treatment Pathway Planning:
- Recommendations for lifestyle modifications.
- Simple interventions (e.g., ovulation induction, IUI) or direct progression to ART (e.g., IVF/ICSI) based on findings.
Red Flags for Immediate ART:
- Severe male factor infertility (e.g., azoospermia).
- Irreparable tubal damage or blockage.
- Advanced maternal age or diminished ovarian reserve.
- Genetic conditions requiring preimplantation genetic testing (PGT).
Thoroughness:
- Comprehensive history-taking and physical examination increase the likelihood of identifying correctable issues.
Collaborative Counseling:
- Ensuring both partners are informed and involved in the decision-making process.
Time Management:
- Quick identification of conditions requiring urgent ART, especially in cases of advanced maternal age.

Counseling and Planning
2) Embryo Transfer Strategy: Single or multiple embryo transfer based on patient age, embryo quality, and desire to avoid multiples.
3) Use of Donor Gametes or Surrogacy: Based on medical necessity and ethical or cultural considerations.
4) Cycle Planning: Timeline based on patient readiness, work schedules, and menstrual cycle.
5) Financial Feasibility: Choose treatment plans that align with the patient’s financial capacity and insurance coverage.
IVF (In Vitro Fertilization):
- Ideal for tubal factor infertility, male factor infertility, unexplained infertility, or failed IUI.
- Overview of the procedure: ovarian stimulation, egg retrieval, fertilization, and embryo transfer.
ICSI (Intracytoplasmic Sperm Injection):
- Typically recommended for severe male factor infertility.
- Explains how a single sperm is injected directly into an egg.
PGT (Preimplantation Genetic Testing):
- For patients with genetic disorders or recurrent pregnancy loss.
- Testing embryos for genetic abnormalities before implantation.
Donor Eggs/Sperm:
- Suitable for patients with poor ovarian reserve, genetic concerns, or azoospermia.
- Discuss the selection and ethical considerations of using donor gametes.
Surrogacy:
- For patients with uterine factor infertility or inability to carry a pregnancy.
- Explain the legal, medical, and emotional aspects.
Factors Influencing Success:
- Age: Success rates decline with increasing maternal age, especially after 35.
- Embryo Quality: Higher-quality embryos improve chances of implantation.
- Uterine Health: Factors like fibroids, endometrial thickness, and uterine anomalies can affect outcomes.
- Laboratory Standards: Success rates vary by clinic due to lab expertise and equipment.
Statistical Overview:
- Average IVF success rates: ~40% per cycle for women under 35, declining to ~10% for women above 42.
- Higher success rates with donor eggs (~50-60%).
Medical Risks:
- Ovarian Hyperstimulation Syndrome (OHSS):
- Caused by excessive response to ovarian stimulation.
- Symptoms: abdominal pain, bloating, nausea, and in severe cases, blood clots or fluid buildup.
- Multiple Pregnancies:
- Transferring multiple embryos increases the risk of twins/triplets.
- Higher maternal and neonatal complications.
- Ectopic Pregnancy:
- Implantation outside the uterus (~2-5% risk).
Emotional and Psychological Risks:
- Stress, anxiety, and depression due to uncertainty or failed cycles.
- Emotional strain in relationships.
Long-Term Concerns:
- Emerging studies on risks to offspring are generally reassuring but warrant discussion.
Breakdown of Costs:
- IVF Cycle: Costs vary by region but typically range from $10,000 to $20,000 per cycle.
- ICSI: Adds $1,000–$2,000 to an IVF cycle.
- PGT: Costs ~$3,000–$8,000 depending on the number of embryos tested.
- Donor Eggs/Sperm: Additional costs of ~$5,000–$15,000 depending on the source and legal requirements.
- Surrogacy: One of the most expensive options (~$50,000–$100,000, including medical, legal, and surrogate compensation).
Insurance Coverage:
- Clarify what aspects of ART are covered by the patient’s insurance plan.
- Explore financing options, if available.
Psychological Impact:
- The process can be emotionally taxing, especially if the patient experiences multiple failed cycles.
- Anxiety about outcomes, societal pressures, and personal expectations.
Coping Strategies:
- Emphasize the importance of stress management techniques like mindfulness, therapy, or support groups.
- Encourage open communication with partners and family.
Support Networks:
- Provide information about local or online support groups for couples undergoing ART.
Mental Health Counseling:
- Referral to mental health professionals specializing in fertility issues, if needed.

Ovarian Stimulation
- Stimulate the ovaries to produce multiple mature follicles.
- Synchronize follicle growth to optimize egg retrieval.
- Prevent premature ovulation during the stimulation cycle.
- Adjust stimulation protocols based on individual ovarian response.
The choice of stimulation protocol depends on the patient’s age, ovarian reserve, and overall health. Common protocols include:
- Long Protocol (GnRH Agonist Protocol):
- Suppresses the natural menstrual cycle using GnRH agonists (e.g., Leuprolide).
- Hormonal suppression is followed by stimulation with gonadotropins (FSH, LH).
- 2) Antagonist Protocol (GnRH Antagonist Protocol):
- A shorter protocol where GnRH antagonists (e.g., Cetrorelix, Ganirelix) prevent premature ovulation.
- Preferred for patients with diminished ovarian reserve or those at risk of ovarian hyperstimulation syndrome (OHSS).
- 3) Mild Stimulation Protocol:
- Uses lower doses of gonadotropins.
- Aimed at producing fewer but higher-quality eggs.
- Suitable for older patients or those with diminished ovarian reserve.
- 4) Natural Cycle IVF:
- No ovarian stimulation is used, and only the naturally selected dominant follicle is retrieved.
- Suitable for specific patients with contraindications to stimulation drugs.
- FSH (Follicle-Stimulating Hormone): Stimulates follicular growth (e.g., Gonal-F, Menopur).
- HMG (Human Menopausal Gonadotropin): Contains both FSH and LH (e.g., Menopur).
- Dosage is adjusted based on patient response.
2) GnRH Agonists/Antagonists:
Prevent premature ovulation during the stimulation phase.
3) hCG (Human Chorionic Gonadotropin) or GnRH Agonist Trigger:
Used to trigger final follicular maturation before egg retrieval.
4) Supplements:
May include DHEA, CoQ10, or other antioxidants to improve egg quality.
- Tracks follicular growth and development.
- Follicles >18mm are typically considered mature and ready for retrieval.
2) Hormonal Monitoring:
- Estradiol (E2) levels rise as follicles grow.
- Monitoring prevents complications like OHSS or poor response.
- Administered when at least 2–3 follicles reach maturity (>18mm in size).
- Timing: 34–36 hours before egg retrieval.
2) GnRH Agonist Trigger:
- Preferred for patients at high risk of OHSS as it reduces the risk.
- Overstimulation leading to abdominal bloating, fluid retention, and in severe cases, organ complications.
- Risk factors: High estradiol levels, polycystic ovary syndrome (PCOS).
2) Poor Ovarian Response:
- Insufficient follicular development.
- Typically seen in older patients or those with diminished ovarian reserve.
3) Cycle Cancellation:
If too few follicles develop or premature ovulation occurs.
4) Multiple Pregnancies (if excess embryos are transferred).
- Ovarian stimulation medications are one of the most expensive components of IVF.
- Costs vary based on the country, brand, and dosage required but typically range from $1,000 to $5,000 per cycle.
- Hormonal fluctuations can cause mood swings, anxiety, or irritability.
- Frequent monitoring appointments can be stressful and time-consuming.
- Counseling and support groups can help alleviate emotional stress during this stage.
Individualized stimulation protocols improve outcomes and reduce risks.
2) Accurate Monitoring:
Regular ultrasounds and hormone checks ensure proper follicular growth.
3) Experienced Clinicians:
Skilled clinicians can identify and mitigate complications early (e.g., OHSS, poor response).

Egg Retrieval and Fertilization
- Triggered with hCG or GnRH agonist 34–36 hours before retrieval.
- Ensures eggs reach the final stage of development.
2) Patient Instructions:
- Fasting for at least 8 hours before the procedure.
- Avoiding certain medications as instructed by the physician.
- Emotional preparation for sedation or anesthesia.
- Performed in a clinic or hospital setting under sedation or general anesthesia.
- Procedure typically takes 20–30 minutes.
2) Technique:
a) Transvaginal Ultrasound-Guided Aspiration:
- A needle is inserted through the vaginal wall into the ovaries.
- Follicular fluid is aspirated, and eggs are retrieved.
b) Eggs are immediately transferred to the lab for evaluation.
3) Monitoring and Recovery:
- Post-retrieval, patients are observed for a short period before discharge.
- Common side effects: Mild cramping, spotting, or bloating.
- Severe symptoms like heavy bleeding, fever, or severe pain require immediate medical attention.
- Eggs are evaluated for maturity and quality.
- Only mature eggs (metaphase II) are used for fertilization.
2) Sperm Preparation:
- Sperm is collected via ejaculation, surgical retrieval (e.g., TESE or PESA), or thawed if previously frozen.
- Prepared through washing and centrifugation to select motile, high-quality sperm.
3) Fertilization Techniques:
a) Conventional IVF:
- Eggs are incubated with sperm in a petri dish to allow natural fertilization.
b) Intracytoplasmic Sperm Injection (ICSI):
- A single sperm is directly injected into the egg.
- Used for severe male factor infertility or low fertilization rates in previous cycles.
4) Fertilization Check:
- Fertilization is assessed 16–18 hours post-procedure.
- Successful fertilization is indicated by the presence of two pronuclei (zygote).
- Fertilized eggs (zygotes) are cultured in a specialized medium.
- Embryos are monitored for growth and development up to the blastocyst stage (5–6 days post-fertilization).
2) Quality Grading:
- Embryos are graded based on cell number, symmetry, and fragmentation.
- High-quality embryos are prioritized for transfer or freezing.
3) Decision on Next Steps:
- Fresh embryo transfer.
- Cryopreservation for future use.
- Preimplantation genetic testing (if indicated).
- Ovarian Hyperstimulation Syndrome (OHSS):
Rare but serious complication where ovaries swell and fluid accumulates in the abdomen.
- Bleeding or Infection:
Rare complications during the needle aspiration process.
- Incomplete Retrieval:
Some follicles may not yield eggs.
2) Fertilization Risks:
- Fertilization Failure:
Occurs when eggs fail to fertilize, even with ICSI.
- Abnormal Fertilization:
Abnormalities in zygote formation may render embryos non-viable.
- Patient age.
- Ovarian reserve and response to stimulation.
- Sperm quality.
2) Fertilization rates:
- Conventional IVF: ~70–80% of mature eggs fertilize.
- ICSI: ~70–90% of mature eggs fertilize.
Costs typically range between $5,000 and $10,000 depending on the clinic and region.
2) ICSI:
Adds $1,000–$2,000 to the overall cost of treatment.
- Physical discomfort and emotional strain due to hormonal changes.
- Managing expectations for fertilization and embryo quality.
- Support from counselors or peers undergoing ART can help alleviate stress.
Skilled clinicians and embryologists increase the likelihood of success.
2) Careful Monitoring and Tailored Stimulation:
Reduces risks of OHSS and ensures optimal egg quality.
3) Advanced Laboratory Protocols:
High-quality culture media and grading systems improve embryo outcomes.

Embryo Culture and Genetic Testing (if applicable)
- Fertilization occurs (via IVF or ICSI).
- Fertilized eggs (zygotes) are assessed for normal fertilization (presence of two pronuclei).
2) Day 1–2 (Cleavage Stage):
- Embryos are monitored for cell division.
- By Day 2, embryos should ideally have 2–4 cells.
3) Day 3 (Early Embryo Assessment):
- Embryos typically reach the 6–8 cell stage.
- Grading is performed based on:
Number and symmetry of cells.
Fragmentation level.
- Decision Point:
Transfer embryos at this stage or continue to culture.
4) Day 5–6 (Blastocyst Stage):
- Embryos reach the blastocyst stage with two distinct regions:
Inner cell mass (forms the fetus).
Trophectoderm (develops into the placenta).
- Embryos are graded based on expansion, cell mass, and trophectoderm quality.
- Decision Point:
Fresh embryo transfer.
Cryopreservation for future use.
Genetic testing (if applicable).
1) When is Genetic Testing Recommended?
- Advanced maternal age (>35 years).
- Recurrent miscarriages or implantation failures.
- Severe male factor infertility.
- Known genetic disorders in the family.
- Couples undergoing fertility preservation.
2) Types of Genetic Testing:
Preimplantation Genetic Testing for Aneuploidy (PGT-A):
- Screens for chromosomal abnormalities (e.g., Down syndrome).
Preimplantation Genetic Testing for Monogenic Disorders (PGT-M):
- Identifies single-gene disorders (e.g., cystic fibrosis, sickle cell anemia).
Preimplantation Genetic Testing for Structural Rearrangements (PGT-SR):
- Detects structural chromosomal abnormalities (e.g., translocations).
Procedure for Genetic Testing:
- Trophectoderm biopsy:
- A few cells are taken from the outer layer of the blastocyst.
- Inner cell mass (fetus) remains unaffected.
- Biopsied cells are sent for analysis, and embryos are cryopreserved until results are available.
Benefits of Genetic Testing:
- Increases implantation rates by selecting chromosomally normal embryos.
- Reduces miscarriage risk.
- Ensures the transfer of healthy embryos.
Benefits:
- Enables future cycles without repeated ovarian stimulation and egg retrieval.
- Allows for time between testing and transfer, reducing patient stress.
- Not all embryos reach the blastocyst stage.
- High rates of attrition, especially in older patients or those with poor-quality eggs/sperm.
2) Damage During Biopsy:
- Trophectoderm biopsy has minimal risks but may occasionally damage embryos.
3) Unusable Embryos After Testing:
- Some embryos may be found to have genetic abnormalities and cannot be transferred.
4) Emotional Stress:
- Waiting for genetic testing results can be emotionally challenging.
- Genetic testing improves implantation rates by selecting chromosomally normal embryos:
- PGT-A: ~65% implantation success with euploid embryos.
- Lower miscarriage rates with genetically tested embryos.
- Typically included in the overall IVF package.
Genetic Testing:
- Costs vary widely but average $3,000–$8,000 depending on the type and number of embryos tested.
Cryopreservation:
- $500–$1,000 annually for embryo storage.
- Counseling helps prepare patients for potential outcomes (e.g., no normal embryos available for transfer).
- Support groups can provide comfort and shared experiences.
- Advanced culture media, temperature, and gas control.
- Skilled embryologists ensure high embryo survival and development rates.
Timing of Decisions:
- Strategic decisions (e.g., biopsy timing, fresh vs. frozen transfer) improve outcomes.
Appropriate Testing:
- Targeted genetic testing ensures the selection of viable embryos while minimizing costs and risks.

Embryo Transfer
Natural Cycle:
- For women with regular cycles, the endometrium is prepared naturally by monitoring the luteinizing hormone (LH) surge and ovulation.
Medicated Cycle:
- Estrogen and progesterone are administered to simulate the natural hormonal environment for endometrial preparation.
- Endometrial thickness >7mm with a trilaminar pattern is ideal for implantation.
2) Embryo Selection:
- Embryos are selected based on their quality and development stage (e.g., Day 3 cleavage embryos or Day 5–6 blastocysts).
- High-quality embryos with good morphology and minimal fragmentation are prioritized.
3) Counseling the Patient:
- Patients are briefed on the procedure and post-transfer care.
- Emotional support is provided, as this is a highly anticipatory stage.
- Transfer is typically performed on Day 3 (cleavage stage) or Day 5–6 (blastocyst stage) after egg retrieval.
- Blastocyst transfers are preferred due to higher implantation rates.
2) Technique:
- A soft catheter is loaded with the selected embryos in a small volume of culture medium.
- Using ultrasound guidance, the catheter is gently inserted through the cervix into the uterine cavity.
- The embryos are deposited approximately 1–2 cm from the uterine fundus.
3) Duration:
- The procedure is painless, non-invasive, and takes about 10–15 minutes.
- Sedation is not typically required unless the patient has cervical stenosis or extreme anxiety.
4) Embryo Number:
Single or multiple embryos may be transferred depending on:
- Patient age.
- Embryo quality.
- History of prior ART cycles.
- Risk of multiple pregnancies.
- Progesterone supplementation is provided via vaginal, oral, or injectable routes.
- Ensures optimal endometrial receptivity for implantation.
2) Activity Restrictions:
- Patients are advised to avoid heavy lifting, strenuous activities, and stress.
- Bed rest is not required, but a balanced approach to activity is recommended.
3) Monitoring and Testing:
- A blood test for beta-hCG is performed 10–14 days after the transfer to confirm pregnancy.
- Ultrasound is conducted 2–3 weeks later to confirm the presence of a gestational sac and fetal heartbeat.
Rare complications include uterine cramping, spotting, or infection.
2) Multiple Pregnancies:
Transferring multiple embryos increases the risk of twins or higher-order pregnancies, which can lead to complications like preterm birth or gestational diabetes.
3) Failed Implantation:
Not all embryo transfers result in pregnancy due to factors like:
- Poor embryo quality.
- Uterine abnormalities.
- Immunological or hormonal issues.
4) Ectopic Pregnancy:
Rarely, the embryo may implant outside the uterus, such as in the fallopian tube.
Patient Age:
a) <35 years: ~50–55% live birth rate per transfer (using blastocysts).
b) 40 years: ~10–20% live birth rate.
Embryo Quality: Higher-quality embryos have better chances of implantation.
Endometrial Receptivity: An endometrium with a trilaminar pattern >7mm increases success.
Transferring a single high-quality blastocyst achieves pregnancy rates comparable to transferring multiple cleavage-stage embryos, reducing the risk of multiples.
- Ultrasound guidance.
- Specialized catheters or assisted hatching (if needed).
Approximate cost: $1,000–$2,000 per transfer (excluding additional ART services).
- This stage is highly emotional due to its proximity to potential pregnancy.
- Patients may experience stress during the two-week waiting period post-transfer.
Support:
- Counseling is essential to help patients manage expectations and cope with outcomes.
- Encouraging participation in support groups can help alleviate emotional burdens.
High-quality embryos and a receptive endometrium are key to successful implantation.
Precision in Technique:
Skilled clinicians ensure accurate placement of embryos within the uterine cavity.
Tailored Luteal Support:
Appropriate hormonal supplementation improves endometrial receptivity.

Luteal Phase Support and Pregnancy Testing
Why is Luteal Support Necessary?
- In ART cycles, the natural luteal phase is often inadequate due to:
- Suppression of the body's natural hormones by medications (e.g., GnRH agonists or antagonists).
- Disruption of natural ovarian function post-egg retrieval.
- Hormonal supplementation ensures proper endometrial support for implantation and early pregnancy.
Hormonal Supplements Used:
- Progesterone:
- Essential for endometrial maintenance and embryo implantation.
- Forms:
- Vaginal (e.g., suppositories, gels): Commonly used for localized delivery.
- Oral: Easy to administer but less effective.
- Injectable: Intramuscular (IM) or subcutaneous (SC), provides sustained levels.
- Estrogen (if needed):
- Used in medicated or frozen embryo transfer (FET) cycles to optimize endometrial receptivity.
- hCG (Human Chorionic Gonadotropin):
- Occasionally used to support the luteal phase but carries a higher risk of ovarian hyperstimulation syndrome (OHSS).
Duration of Luteal Support:
- Luteal support typically continues until:
- Confirmation of pregnancy (~10–14 days post-embryo transfer).
- If pregnant, hormonal support may continue until 10–12 weeks of gestation (when the placenta takes over hormone production).
Lifestyle Recommendations:
- Avoid high-impact activities and heavy lifting.
- Follow a balanced diet and stay hydrated.
- Manage stress through relaxation techniques like yoga or meditation.
Symptom Monitoring:
- Normal: Mild cramping, breast tenderness, or spotting.
- Seek medical attention if experiencing heavy bleeding, severe pain, or symptoms of OHSS (bloating, nausea, rapid weight gain).
Compliance with Medication:
- Adherence to prescribed progesterone or estrogen regimens is critical.
1) When to Test?
- A blood test for beta-hCG is typically performed 10–14 days after embryo transfer.
- Testing earlier may lead to false-negative results due to low hCG levels.
2) Types of Tests:
- Quantitative Beta-hCG Test:
- Measures the exact level of hCG in the blood.
- Levels >25 mIU/mL are considered positive for pregnancy.
- Qualitative Pregnancy Test:
- A simple "yes/no" blood or urine test.
- Less sensitive than quantitative testing.
3) Interpreting Results:
- Positive:
- Indicates successful implantation.
- hCG levels should double approximately every 48–72 hours in early pregnancy.
- Negative:
- Indicates no implantation.
- Further evaluation of cycle outcome may be necessary.
4) Next Steps After Positive Test:
- Ultrasound is scheduled 2–3 weeks after a positive test to confirm:
- Gestational sac.
- Fetal heartbeat.
- Luteal support may continue as prescribed.
Hormonal Side Effects:
- Progesterone: Breast tenderness, bloating, fatigue, or mild cramping.
- Estrogen: Nausea, headaches, or fluid retention.
Emotional Stress:
- The "two-week wait" between transfer and pregnancy testing can cause significant anxiety.
- Patients often experience heightened emotions during this phase.
Failed Implantation:
- If pregnancy is not achieved, it may indicate:
- Poor embryo quality.
- Uterine abnormalities.
- Hormonal insufficiency.
- Counseling and review of the cycle are critical.
- Adherence to Luteal Support Protocols:
- Consistent medication use ensures optimal endometrial receptivity.
- Early Monitoring and Adjustments:
- Close monitoring of hormone levels can prevent complications like luteal phase deficiency.
- Supportive Care:
- Counseling during the two-week wait helps patients manage expectations and emotional stress.
The wait for pregnancy test results can be one of the most emotionally taxing periods in ART.
Support Systems:
Family, friends, or support groups can provide reassurance.
Professional Counseling:
Fertility counselors can guide patients through both positive and negative outcomes.
Luteal phase support is usually included in the overall ART package but may add additional costs for medications:
- Progesterone: $200–$600 per cycle.
- Estrogen: $50–$150 per cycle.
- Lifestyle and activity recommendations to optimize implantation.
- Pregnancy testing with beta-hCG 10–14 days post-transfer.
- Monitoring for side effects or complications like OHSS.
- Emotional and counseling support during the waiting period.
